
:max_bytes(150000):strip_icc()/PeriodicTable_AtomSizes-56a131193df78cf772684720.png)
When two atoms combine, we can possibly determine the size of atoms by checking the distance between them. Atomic size is the distance between the atoms outermost shell and that to the center of the nucleus. Compare electron affinities and electronegativities. Electrons are negatively charged and attracted towards protons. Predict greater or smaller atomic size and radial distribution in neutral atoms and ions. Referring only to a periodic table and not to Figure 8.19 Atomic Radii Trends on the Periodic Table, which atom. There are more protons in atoms moving down a group (greater positive charge), yet the effect is to pull in the electron shells, making them smaller and screening outer electrons from the attractive force of the nucleus. Proton is positively charged, and the neutron is neutral. This is because the principal quantum number of the outermost electron increases moving down a group. But there are some exceptions observed such as the atomic radius of the oxygen atom is. Ionization decreases moving top to bottom down an element group (column). From left to right, the period size of the atoms generally decreases.The noble gas has a filled valence shell, so it resists electron removal. Consequently, the size of the atom (and its covalent radius) must increase as we increase the distance of the outermost electrons from the nucleus. The atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. (b) Covalent radii of the elements are shown to scale. The atomic radius for the halogens increases down the group as n increases. Ionization is at its minimum value for the alkali metal on the left side of the table and a maximum for the noble gas on the far right side of a period. 1: (a) The radius of an atom is defined as one-half the distance between the nuclei in a molecule consisting of two identical atoms joined by a covalent bond. Element \text B B has more electron-occupied shells than element \text A A. This time, even though the number of protons increases by a lot, the electron valence shells do not. Again, this is due to the effective charge at the nucleus.
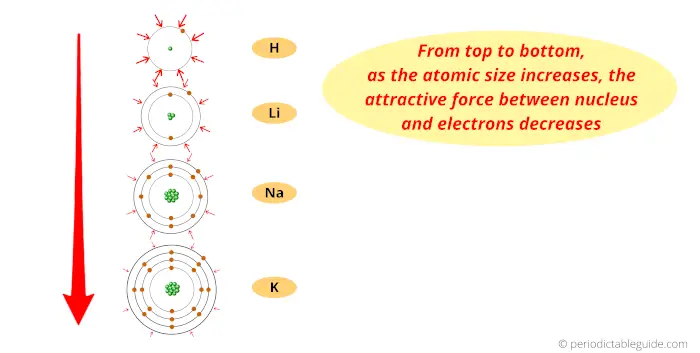
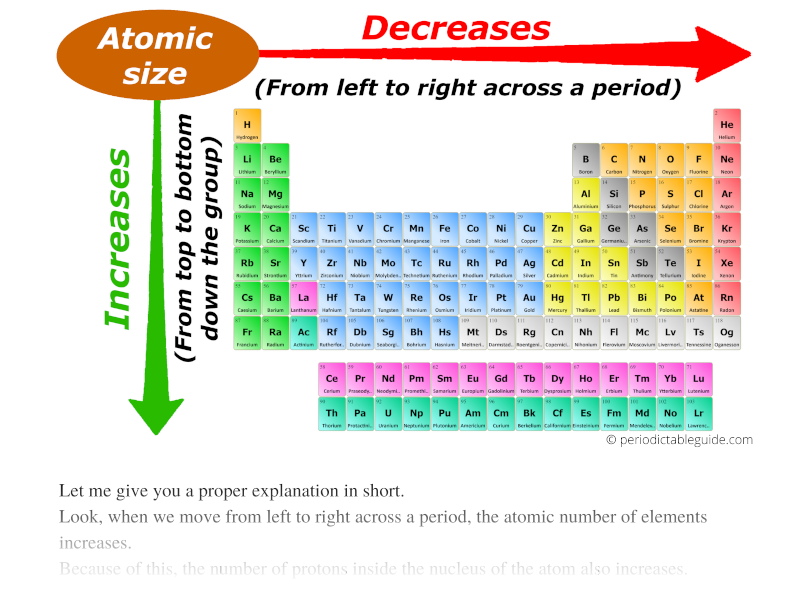
This direction increases the size of the atom. Well this was just an introduction about the atomic radius trends (or atomic size trends) in periodic table. Elements \text A A & \text B B belong to the same group. The other trend of atomic radius or atom size occurs as you move vertically down an element group. This is because the atomic radius generally decreases moving across a period, so there is a greater effective attraction between the negatively charged electrons and positively-charged nucleus. The atomic radius (or atomic size) of the elements decreases as we move across a period (from left to right) and it increases as we move down in a group (from top to bottom). Ionization energy generally increases moving from left to right across an element period (row).Thus, it takes much more energy than just overcoming a larger ionic charge would suggest.
Why is it so much larger? Because the first two electrons are removed from the 3 s subshell, but the third electron has to be removed from the n = 2 shell (specifically, the 2 p subshell, which is lower in energy than the n = 3 shell). The third IE, however, is over five times the previous one. The second IE is twice the first, which is not a surprise: the first IE involves removing an electron from a neutral atom, while the second one involves removing an electron from a positive ion.


 0 kommentar(er)
0 kommentar(er)
